
Histomoniasis, generally often called blackhead illness, is brought on by an anerobic protozoan parasite, Histomonas meleagridis. Histomoniasis impacts all gallinaceous birds and turkeys are probably the most susceptible species. Lately, elevated incidences of histomoniasis have been documented in chickens.
Vijay Durairaj1, Mary Drozd2, Emily Barber1, Brandon Doss3, Ryan Vander Veen1
1 Huvepharma, Inc., Lincoln, Nebraska, USA
2 College of Nebraska-Lincoln, Lincoln, Nebraska, USA
3 Huvepharma, Inc., Peachtree Metropolis, Georgia, USA
Corresponding creator: Vijay.Durairaj@huvepharma.us
A case report of histomoniasis in a four-week-old-broiler breeder pullet farm is mentioned right here. On discipline investigation, gross lesions in ceca and liver have been seen suggesting histomoniasis. Throughout histopathological analysis, Histomonas trophozoites have been recognized. H. meleagridis and Blastocystis spp. have been remoted in tradition. Superior molecular diagnostic strategies confirmed genotype-1 H. meleagridis. With the absence of business vaccines and prophylactic/therapeutic measures, discipline surveillance research play a significant position in understanding H. meleagridis wild-type strains and figuring out options for histomoniasis.
Introduction
Histomoniasis was first reported in turkeys by Cushman (1893). Histomonas meleagridis, an anerobic protozoan parasite, causes histomoniasis (histomonosis, enterohepatitis, enzootic typhlohepatitis). H. meleagridis impacts all gallinaceous birds and turkeys are extremely vulnerable. Heterakis gallinarum, the frequent cecal worm of chickens, acts as a vector for transmitting H. meleagridis. Heterakis gallinarum eggs can harbor H. meleagridis for a number of years. Thus, it isn’t advisable to rear chickens and turkeys on the identical premise or in shut proximity to 1 one other.
Among the many gallinaceous species, turkeys are extremely vulnerable to histomoniasis. In turkeys, the mortalities can attain as much as 80%-100% (Hess, 2020). Chickens mount a greater immune response to H. meleagridis in comparison with turkeys (Powell et al., 2009; Mitra et al., 2017). Thus, the severity of histomoniasis will not be as pronounced in chickens and will induce mortality as much as 10%-20% (McDougald, 2005). Based mostly on 1986 AAAP Committee on Illness Reporting, the financial losses related to histomoniasis are extra vital in chickens in comparison with turkeys as a result of variety of birds concerned and frequency of incidences (Callait, 2002). Lately, elevated incidences of histomoniasis have been documented in chickens.
Prior to now, histomoniasis was managed and managed by prophylactic remedy with Arsenics (i.e Carbarsone and Nitarsone), and therapeutic remedy with Nitro-imidazoles (Dimetridazole, Iprondidazole) and Nitrofurans (Furazolidone, Salfuride) (Clark, 2017). As a consequence of varied causes, these merchandise have been eliminated/withdrawn from the market. At current, there aren’t any industrial vaccines out there to fight histomoniasis. In sure nations, just a few therapeutic/ prophylactic merchandise are used to fight histomoniasis, however the efficacy of those merchandise are extremely variable.
With this set of circumstances, the prevention of histomoniasis transmission by following strict biosecurity measures helps in minimizing the chance of spreading the illness. Area surveillance research support in understanding the present H. meleagridis wild-type isolates and to identification potential options for histomoniasis.
Supplies and strategies
Case historical past
In Spring 2022, histomoniasis was reported in two out of seven homes (n=14,000 birds/home) in four-week-old-broiler breeder pullets in South Central, USA. Elevated mortality was reported in each homes. On necropsy, gross lesions have been seen within the ceca and liver. Ceca and liver samples have been collected in 10% impartial buffered formalin for histopathological analysis. Further cecal samples have been collected in plug seal-capped flasks containing 10 ml of modified Dwyer’s media (Hauck, 2010) and positioned in a heat insulated Styrofoam field and safely transported to the lab. As well as, entire intestines have been collected and shipped in a chilly insulated Styrofoam field.
Histopathology
Necropsy tissues have been instantly mounted in 10% impartial buffered formalin. Following fixation, sections have been processed routinely, paraffin-embedded and sectioned at 3-4 μm and stained with hematoxylin-eosin. Histologic tissues have been evaluated by a board-certified pathologist.
Tradition
The plug seal-capped flasks with cecal samples have been obtained in heat situation. The flasks have been supplemented with recent pre-warmed modified Dwyer’s media (5 ml) after which moved to an incubator (40°C) and maintained in anaerobic situation. Periodically, the cultures have been noticed below inverted microscope.
DNA extraction and PCR
Intestinal samples have been evaluated and a chunk of cecal tissue was homogenized with glass beads. DNA was extracted from the homogenized cecal pattern utilizing DNeasy Blood & Tissue Equipment (Qiagen, Hilden, Germany) following the producer’s directions. Every 50 µL PCR response consisted of 1X GoTaq G2 Sizzling Begin Inexperienced Grasp Combine (Promega, Madison, WI), 0.2 µM of every primer, and 5 µL of template. PCR was carried out utilizing protozoa 18s rRNA primers (9. Bilic 2014), H. meleagridis rpb1 gene particular primers (9. Bilic 2014), SSU rRNA of Blastocystis primers (10. Hess et al., 2006), and mtCOI gene of Eimeria sp. primers together with the described biking situations.
Gel electrophoresis of PCR merchandise and sequencing
The amplicons (2 µL) have been visualized utilizing E-Gel™ EX Agarose Gels, 2% (Invitrogen, Carlsbad, CA) with E-Gel™ 1 Kb Plus DNA Ladder (Invitrogen™). The QIAquick PCR purification package (Qiagen) was used to purify the PCR merchandise for sequencing (Eurofins, Louisville, KY).
Outcomes
Gross pathology
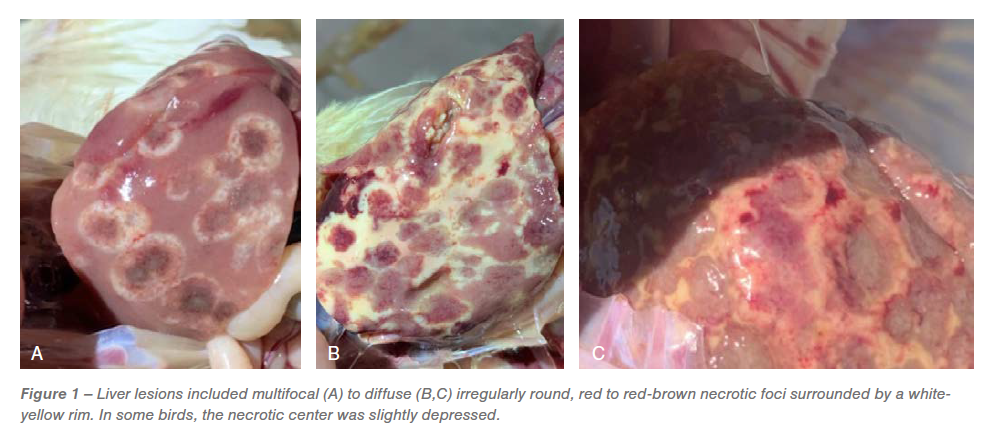
Gross lesions within the ceca included typhlitis and cecal cores. The liver lesions had quite a few darkish crimson centered multifocal necrotic foci surrounded by white periphery resembling bulls-eye (Determine 1A), diffuse irregularly spherical, crimson necrotic foci surrounded by white-yellow rim, leading to discoloration of the liver (Determine 1B) and red-brown necrotic foci leading to slight depressions on the floor of the liver resembling saucer formed lesions (Determine 1C). Based mostly on the distinctive gross lesions within the ceca and liver, a presumptive analysis of histomoniasis was made.
 Histopathology
Histopathology
Ceca: Roughly 60% to 95% of the mucosa was changed by predominately granulomatous irritation and granulation tissue infiltrated by pleomorphic micro organism and Histomonas trophozoites (Figures 2 and three). The luminal floor was lined in a fibrinocellular exudate infiltrated by protozoa and micro organism. The underlying submucosa was effaced by histiocytic to pyogranulomatous irritation and granulation tissue that’s infiltrated by trophozoites (Figures 2 and three). 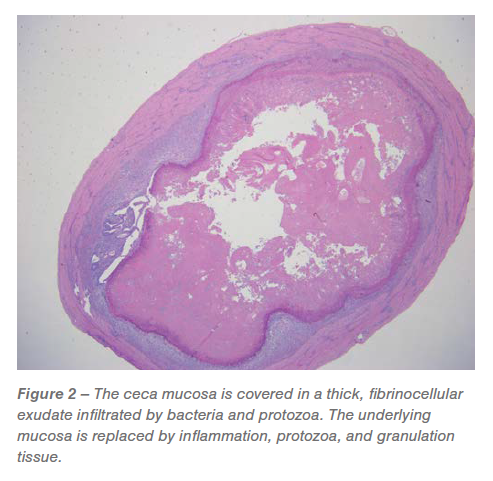
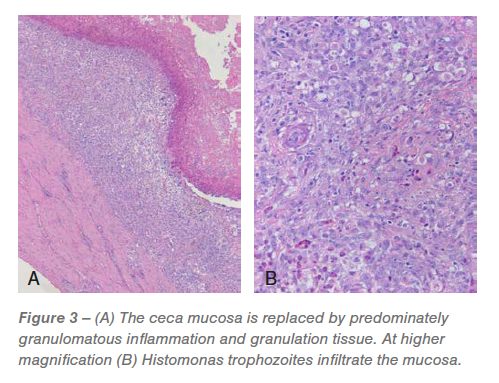
The remaining mucosa had plentiful lamina propria fusion and irritation, bacterial and protozoa, crypt loss and in depth, irregular distention of the remaining crypts with protein and mobile particles. The tunica muscularis was expanded and mildly effaced by multifocal to coalescing, predominantly histiocytic irritation that’s most prevalent round small blood vessels and sometimes has a central nidus of necrosis and trophozoites. The related mesentery was thickened by lymphohistiocytic irritation.
Liver: Between 20% and 75% of the examined liver sections have been severely effaced by multifocal to coalescing, random, hepatocellular necrosis infiltrated by histiocytic and combined irritation with granuloma group, variable numbers of trophozoites and protein (Determine 4). 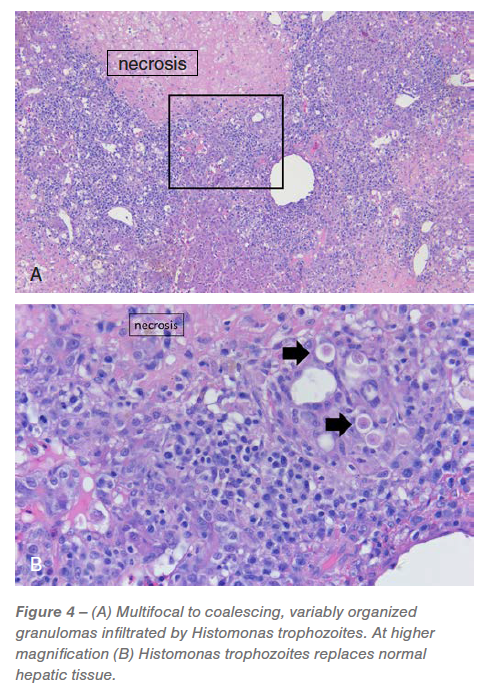
Tradition
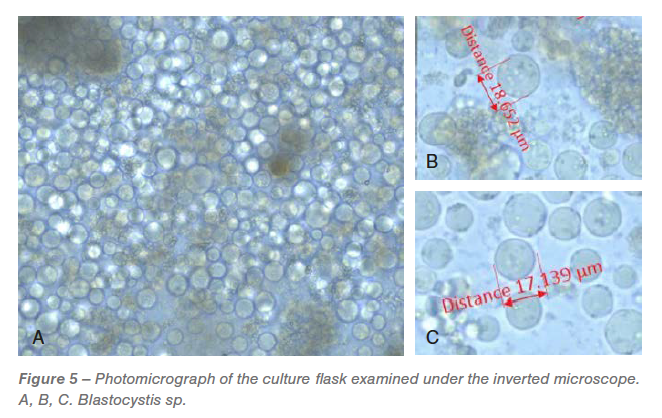
On the day after receipt, all of the flasks have been examined below the microscope. An overwhelmed Blastocystis spp. inhabitants (Determine 5) with various sizes have been noticed in all of the flasks. Two days after incubation, H. meleagridis have been detected within the tradition however have been troublesome to doc by microscopic pictures because of overwhelming progress of Blastocystis spp. All of the flasks had unidentified bacterial inhabitants.
 PCR and sequencing
PCR and sequencing
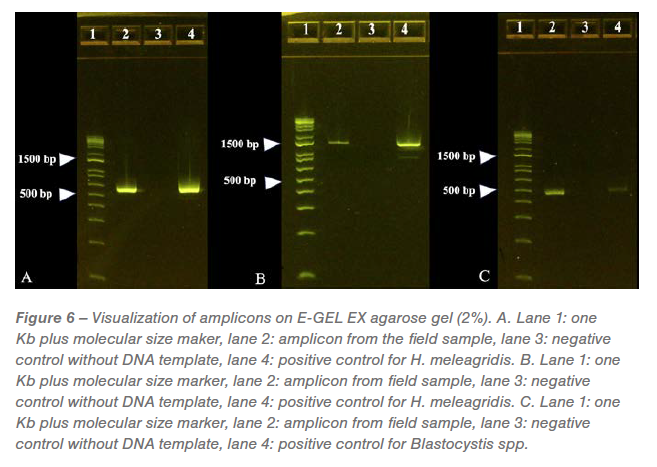
The expected measurement of every amplicon generated from the PCR reactions was visualized on separate E-Gels. A optimistic band was seen at roughly 550 bp within the amplicons generated by PCR directed in opposition to protozoal 18s RNA and confirmed as genotype-1 H. meleagridis with 95.17% identification to wild-type H. meleagridis (Determine 6A). A optimistic band was seen at roughly 1240 bp within the amplicons generated by PCR directed in opposition to H. meleagridis Rpb1 gene and confirmed as genotype-1 H. meleagridis with 99.66% identification to wild-type H. meleagridis (Determine 6B). A optimistic band was seen at roughly 500 bp within the amplicons generated by PCR directed in opposition to Blastocystis spp. with 99.30% identification to wild-type Blastocystis spp. (Determine 6C). No bands have been noticed on Eimeria sp. particular PCR (gel picture not proven).
 Dialogue
Dialogue
Histomoniasis in chickens was sometimes reported prior to now. Withdrawal and ban of prophylactics/therapeutics in poultry has resulted in elevated circumstances of histomoniasis. The variety of birds concerned, together with elevated incidences and accompanying morbidity and mortality, has positioned histomoniasis as an economically necessary illness within the rooster trade. Within the USA, a day-old broiler breeder feminine chick prices ~ $10 and male chick prices ~$14. Contemplating the feeding, administration and labor prices at four-weeks-of-age, a broiler breeder pullet prices roughly $13 to $17. For instance, in a flock of 14,000 birds at four-weeks-of-age, if 1% of elevated mortality is attributable to histomoniasis, it’ll lead to extra losses of $1820-$2380. If the mortality related to histomoniasis elevated to five%, it’ll lead to a further lack of $9,100 to $11,900.
A presumptive analysis of histomoniasis was made based mostly on the attribute gross lesions within the ceca and liver. On this case, the liver lesions have been distinctive suggesting histomoniasis. In some situations, H. meleagridis doesn’t trigger typical lesions within the liver. In different situations, the liver and cecal lesions could also be induced by totally different pathogens. Lesions within the rooster liver will be induced by parasites equivalent to H. meleagridis, Tetratrichomonas gallinarum and Leucocytozoon caulleryi, viruses equivalent to Marek’s illness virus, Avian leukosis virus, reticuloendothelial virus, fowl adenovirus-1, avian hepatitis E virus and micro organism equivalent to Salmonella pullorum, Salmonella gallinarum, Pasteurella multocida, Mycobacterium avian, Enterococcus cecorum, Streptococcus gallolyticus subsp. gallolyticus, Escherichia coli (perihepatitis), Mycoplasma gallisepticum (perihepatitis), Mycoplasma synoviae (perihepatitis), Clostridium perfringens, Camplylobacter hepaticus, Staphylococcus, and Erysipelothrix rhusiopathiae. Different situations that may trigger liver lesions embrace hemorrhagic hepatopathy, fatty liver hemorrhagic syndrome, aflatoxins, amyloidosis, ascites, visceral gout, warmth stress and excessive vitality weight-reduction plan. Lesions within the rooster ceca will be induced by parasites equivalent to H. meleagridis, Eimeria tenella, Tetratrichomonas gallinarum and micro organism equivalent to Salmonella sp. Mixed liver and cecal lesions in chickens will be induced by H. meleagridis, Tetratrichomonas gallinarum and Salmonella Sp.
Histology of the ceca and liver confirmed H. meleagridis as the reason for necroulcerative typhilitis and necrotizing and granulomatous hepatitis in these chickens.
The cecal tradition incubated in modified Dwyer’s media had overwhelming progress of Blastocystis spp. of varied sizes. Blastocystis spp. is a typical protozoan parasite that’s current within the gut of the chickens (Grabensteiner et al., 2006; Chadwick et al., 2000). Though debatable, Blastocystis sp. doesn’t have an enormous medical influence (Chadwick et al., 2000; Stensvold et al., 2009).
H. meleagridis has two genotypes, particularly genotype 1 and a couple of (Bilic et al., 2014). The pathological manifestations and the medical indicators fluctuate between the genotypes. PCR and sequencing confirmed that the wild-type H. meleagridis reported on this research was genotype 1. In Europe, genotype 1 is predominant, whereas genotype 2 is uncommon (Bilic et al., 2014). Within the USA, based mostly on our discipline surveillance over the previous few years, genotype 1 was recognized in all the sphere outbreaks studied. Superior molecular strategies equivalent to PCR and sequencing helps present helpful insights on the genotype degree. Thus, discipline surveillance research assist in understanding present H. meleagridis wild-type isolates and will be helpful in figuring out an answer for histomoniasis.
Acknowledgment
The authors wish to thank the poultry firm and the supervisor for collaborating on this discipline case.
References
- Cushman, S. The manufacturing of turkeys. In: Bulletin 25, Agricultural Experiment Station, Rhode Island School of Agriculture and Mechanical Arts, Kingston, RI. 89–123. 1893.
- Hess M., McDougald LR. Histomoniasis. In: Swayne D, Boulianne M, Logue C, McDougald L, Nair V., Suarez D., deWit S., Grimes T., Johnson D., Kromm M., et al., editors. Ailments of Poultry. 14th ed. Ames (IA): Wiley- Blackwell. 1223–1230; 2020.
- Powell, F. L., L. Rothwell, M. J. Clarkson, and P. Kaiser. The turkey, in comparison with the rooster, fails to mount an efficient early immune response to Histomonas meleagridis within the intestine. Parasite Immunol. 31:312–327. 2009.
- Mitra, T., W. Gerner, F. A. Kidane, P. Wernsdorf, M. Hess, A. Saalmuller, and D. Liebhart. Vaccination in opposition to histomonosis limits pronounced adjustments of B cells and T-cell subsets in turkeys and chickens. Vaccine 35:4184–4196. 2017.
- McDougald LR. Blackhead illness (histomoniasis) in poultry: a vital assessment. Avian Dis. 49:462–476; 2005.
- Callait, M. P., C. Granier, C. Chauve, and L. Zenner. In vitro exercise of therapeutic medicine in opposition to Histomonas meleagridis (Smith, 1895). Poult. Sci. 81:1122–1127. 2002.
- Clark S, Kimminau E. Vital assessment: future management of blackhead illness (histomoniasis) in poultry. Avian Dis. 61:281–288; 2017.
- Hauck R, Armstrong PL, McDougald LR. Histomonas meleagridis (Protozoa: Trichomonadidae): evaluation of progress necessities in vitro. J Parasitol. 96:1–7; 2010.
- Bilic I, Jaskulska B, Souillard R, Liebhart D, Hess M. Multi-locus typing of Histomonas meleagridis isolates demonstrates the existence of two totally different genotypes. PLOS ONE 9:e92438; 2014.
- Hess, M., T. Kolbe, E. Grabensteiner, and H. Prosl. Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. established by micromanipulation. Parasitology. 133:547–54. 2006.
- Grabensteiner, E., and M. Hess. PCR for the identification and differentiation of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis spp. Vet. Parasitol. 142:223–230. 2006.
- Chadwick E, Malheiros R, Oviedo E, Cordova Noboa HA, Quintana Ospina GA, Alfaro Wisaquillo MC, Sigmon C, Beckstead R. Early an infection with Histomonas meleagridis has restricted results on broiler breeder hens’ progress and egg manufacturing and high quality. Poult Sci. 99:4242-4248. 2020.
- Stensvold CR, Alfellani MA, Nørskov-Lauritsen S, Prip Okay, Victory EL, Maddox C, Nielsen HV, Clark CG. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a brand new subtype. Int J Parasitol. 39:473-9. 2009.


